Learning Objective
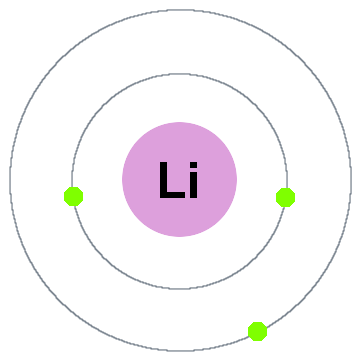
Helium is located in period 1, group 18 of the Periodic Table and has an atomic number equal to 2. As a result, neutral helium will only have 2 electrons surrounding its nucleus. Valence electrons are the electrons located in an atom's outermost shell. In helium's case, both its electrons will be valence electrons. For Groups 13 through 18, the number of valence electrons is the group number minus 10. The exception to this rule is helium (He). Helium is in Group 18. However, helium atoms have only two electrons, so they have two valence electrons, rather than eight.
1. Draw a Lewis electron dot diagram for an atom or a monatomic ion.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does not matter what order the positions are used.) For example, the Lewis electron dot diagram for hydrogen is simply
Because the side is not important, the Lewis electron dot diagram could also be drawn as follows:

The electron dot diagram for helium, with two valence electrons, is as follows:
By putting the two electrons together on the same side, we emphasize the fact that these two electrons are both in the 1s subshell; this is the common convention we will adopt, although there will be exceptions later. The next atom, lithium, has an electron configuration of 1s22s1, so it has only one electron in its valence shell. Its electron dot diagram resembles that of hydrogen, except the symbol for lithium is used:
Beryllium has two valence electrons in its 2s shell, so its electron dot diagram is like that of helium:
The next atom is boron. Its valence electron shell is 2s22p1, so it has three valence electrons. The third electron will go on another side of the symbol:
Again, it does not matter on which sides of the symbol the electron dots are positioned.
For carbon, there are four valence electrons, two in the 2s subshell and two in the 2p subshell. As usual, we will draw two dots together on one side, to represent the 2s electrons. However, conventionally, we draw the dots for the two p electrons on different sides. As such, the electron dot diagram for carbon is as follows:
With nitrogen, which has three p electrons, we put a single dot on each of the three remaining sides:
For oxygen, which has four p electrons, we now have to start doubling up on the dots on one other side of the symbol. When doubling up electrons, make sure that a side has no more than two electrons.
Fluorine and neon have seven and eight dots, respectively:
With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell, the n = 3 shell. By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
Example 1
What is the Lewis electron dot diagram for each element?
- aluminum
- selenium
Solution
The valence electron configuration for aluminum is 3s23p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons:
The valence electron configuration for selenium is 4s24p4. In the highest-numbered shell, the n = 4 shell, there are six electrons. Its electron dot diagram is as follows:
Test Yourself
What is the Lewis electron dot diagram for each element?
- phosphorus
- argon
Answer
For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s23d6) is as follows:
Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Thus the electron dot diagrams for the first column of elements are as follows:
Monatomic ions are atoms that have either lost (for cations) or gained (for anions) electrons. Electron dot diagrams for ions are the same as for atoms, except that some electrons have been removed for cations, while some electrons have been added for anions. Thus in comparing the electron configurations and electron dot diagrams for the Na atom and the Na+ ion, we note that the Na atom has a single valence electron in its Lewis diagram, while the Na+ ion has lost that one valence electron:
Technically, the valence shell of the Na+ ion is now the n = 2 shell, which has eight electrons in it. So why do we not put eight dots around Na+? Conventionally, when we show electron dot diagrams for ions, we show the original valence shell of the atom, which in this case is the n = 3 shell and empty in the Na+ ion.
In making cations, electrons are first lost from the highest numbered shell, not necessarily the last subshell filled. For example, in going from the neutral Fe atom to the Fe2+ ion, the Fe atom loses its two 4s electrons first, not its 3d electrons, despite the fact that the 3d subshell is the last subshell being filled. Thus we have
Anions have extra electrons when compared to the original atom. Here is a comparison of the Cl atom with the Cl− ion:
Example 2
What is the Lewis electron dot diagram for each ion?
- Ca2+
- O2−
Solution
Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca2+.
Ca2+
The O2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows:
Test Yourself
The valence electron configuration of thallium, whose symbol is Tl, is 6s25d106p1. What is the Lewis electron dot diagram for the Tl+ ion?
Answer
Key Takeaways
- Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol.
- Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom.
Exercises
Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol.
Is it necessary for the first dot around an atomic symbol to go on a particular side of the atomic symbol?
What column of the periodic table has Lewis electron dot diagrams with two electrons?
What column of the periodic table has Lewis electron dot diagrams that have six electrons in them?
Draw the Lewis electron dot diagram for each element.
a) strontium
b) silicon
6. Draw the Lewis electron dot diagram for each element.
a) krypton
b) sulfur
7. Draw the Lewis electron dot diagram for each element.
a) titanium
b) phosphorus
8. Draw the Lewis electron dot diagram for each element.
a) bromine
b) gallium
9. Draw the Lewis electron dot diagram for each ion.
a) Mg2+
b) S2−
10. Draw the Lewis electron dot diagram for each ion.
a) In+
b) Br−
11. Draw the Lewis electron dot diagram for each ion.
a) Fe2+
b) N3−
12. Draw the Lewis electron dot diagram for each ion.
a) H+
b) H−
Answers
1.
The first two electrons in a valence shell are s electrons, which are paired.
3.
the second column of the periodic table
5.
a)
b)
7.
a)
b)
9.
a) Mg2+
b)
11.
a) Fe2+
b)
Aluminum Valence Electrons
Electron Configuration
The electrons in an atom fill up its atomic orbitals according to theAufbau Principle; 'Aufbau,' in German, means 'building up.' The AufbauPrinciple prescribes a few simple rules to determine the order atomicorbitals are filled with electrons:
- Electrons always fill orbitals of lower energy first. 1s isfilled before 2s, and 2s before 2p.
- If two electrons occupy the same orbital, they must have opposite spin, as required by the Pauli Exclusion Principle.
- When electrons have to choose between two or more orbitals of thesame energy, electrons prefer to go into different orbitals. As more electrons as added to the atom, these electrons tend to half-fill orbitals of the same energy before pairing with existing electrons to fill orbitals. This isknown as Hund's Rule.
Valency and Valence Electrons
The outermost shell of an atom is its valence shell, and the electrons in the valence shell are valence electrons. Valence electrons are the highest energy electrons in an atom and are therefore the most reactive. While inner electrons (those not in the valence shell) typically don't participate in chemical bonding and reactions, valence electrons can be gained, lost, or shared to form chemical bonds. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table.
The electron configurations for the first and second row elements are shownin in simplified notation.
The Octet Rule
Our discussion of valence electron configurations leads us to one of thecardinal tenets of chemical bonding, the octet rule. The octet rulestates that atoms become especially stable when their valence shells gain afull complement of valence electrons. For example, in above, Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble gases, exist in free atomic form and do not usually form chemical bonds with other atoms.
Helium Valence Electrons Count
Most elements, however, do not have a full outer shell and are too unstableto exist as free atoms. Instead they seek to fill their outer electronshells by forming chemical bonds with other atoms and thereby attain Noble Gas configuration. An element will tend to take the shortest path to achieving Noble Gas configuration, whether that means gaining or losing one electron. For example, sodium, which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon. When two different elements have the same electron configuration, they are called isoelectronic.
